The UK coast is home to many different bird species, which play a key role in the coastal ecosystems. They depend, directly and indirectly, on the marine and coastal environment. Seabirds are often used as bioindicators of marine ecosystems as they are easy to detect and survey, and are top predators; their presence and abundance can indicate the health and status of the habitat and food chain.
This guide contains some key identification features to look out for while out birdwatching by the coast. It is possible to see birds along our coastline throughout the year, but in late spring and early summer, particularly around June, many seabird species are feeding chicks. This is, therefore, the best time of year to spot some of our iconic coastal birds.
Very little equipment is needed for birdwatching, but it is generally recommended to bring a pair of binoculars or a scope, as this helps to see the less obvious features that aid in identifying species. These also allow you to observe without getting too close and disturbing the wildlife. A pen and notebook to keep a record of the species you spot is also a good idea, along with a field guide for the species not mentioned on this list.
Herring gull (Larus argentatus)
Size: length (L): 54-60 cm, wingspan (WS): 123-148cm
A widespread and common (though declining) gull species across the UK. They are large white birds with grey back and wings (tipped with black), a yellow bill and pink legs, and can be seen far inland in almost any habitat from the coast to farmland, moorland, town and city. This highly versatile species is our archetypal ‘seagull’.

Lesser black-backed gull (Larus fuscus)
Size: L: 48-56cm, WS: 117-134cm
Slightly smaller than the herring gull with darker slate-grey back and wings. Look out for yellow legs which are a key identifier of this species. Favours rocky coasts but is also found inland in mixed flocks with other gulls and around inland lakes.

Great black-backed gull (Larus Marinus)
Size: L: 61-74cm, WS: 144-166cm
These are very large and stocky gulls with a dark grey back and wings, a thick-set yellow bill and pink legs. This species are far more prominent along coastal areas and nest largely on rocky islands.

Black-headed gull (Chroicocephalus ridibundus)
Size: L: 35-39cm, WS: 86-99cm
Another widespread and common species often found inland, black-headed gulls have white heads for most of the year, often with a prominent black ear spot. In the summer, adult birds heads turns a dark chocolaty brown. They also have a red bill and red legs.

Kittiwake (Rissa tridactyla)
Size: L: 37-42cm, WS: 93-105 cm
A somewhat slight looking gull, white bodied with pale grey back and wings and black wing tips. They have a small yellow bill, dark eyes and black legs. Predominantly a summer breeding species in the UK and very rarely seen away from the coast, though they are known to form colonies within urban areas near ports.

Common tern (Sterna hirundo)
Size: L: 34-37cm, WS: 70-80 cm
Slight, slender and angular birds, often breeding in colonies around coastal lakes and lagoons, common terns are bright white with a light grey back and wings, a deeply forked tail, black cap and red legs and bill. Their bill has a black tip.

Arctic tern (Sterna paradisaea)
Size: L:33-39cm, WS: 66-77 cm
For much of the UK Arctic terns will be spotted on passage during their incredibly long migration. They are superficially similar to common tern though their bill is usually plain red with no black tip.

Fulmar (Fulmarus glacialis)
Size: L: 43-52cm, WS: 101-117 cm
Fulmar, though gull like in appearance, are petrels, related to albatrosses and shearwaters. They have a thick-set ‘tube-nose’ bill which they can spit foul-smelling oil from to deter predators from their nesting sites. They nest on sheer cliff faces and fly on stiff and shallow wing beats.

Gannet (Morus bassanus)
Size: L: 85-97cm, WS: 170-192 cm
Gannets are large white seabirds with a distinctive yellow head and long pointed wings with black tips. They also have a long pointed grey bill and white pointed tale. They can be seen flying high over the sea and circling before plunging at great speeds into the water in pursuit of food.

Guillemot (Uria aalge)
Size: L: 38-46cm, WS: 61-73 cm
Guillemots come to land only in the summer to breed and do so in large colonies on sheer cliff faces. Adult birds can often be seen ‘rafting’ at sea below the colony also. They have a brown/black head, back, wings and tail and white underneath. There is also a ‘bridled’ form where the birds have a white ring around their eye with a stripe behind it.

Razorbill (Alca torda)
Size: L: 38-43cm, WS: 60-69 cm
Razorbills are superficially similar to guillemot: black on their wings, back, head and tail and white underneath. An easy distinction between the species can be made however, by the razorbills deep thick-set blunt bill, where the guillemot has a longer slim bill. They are another summer breeder, wintering in the northern Atlantic, and favouring sheer rocky cliffs and islands for nesting.

Puffin (Fratercula arctica)
Size: L: 28-34cm, WS: 50-60 cm
Unmistakeable small seabirds with a black back and white underneath. They have a white face with dark eyes set in dark triangular markings and an iconic vibrantly colourful bill. They are a summer visitor predominantly in large nesting colonies on islands, where they nest in burrows along vast grassy banks.

Cormorant (Phalacrocorax carbo)
Size: L: 77-94cm, WS: 121-149 cm
A large long-necked black bird, with a white face and yellow and grey bill. Cormorants are often seen inland on rivers and lakes, and in harbours where they extensively dive for their food and then stand to dry with their wings characteristically spread wide.

Shag (Phalacrocorax aristotelis)
Size: L: 68-78cm, WS: 95-110 cm
Shags are similar in appearance to cormorants though smaller, with a slimmer bill. Adult birds are entirely black (lacking the white face of cormorants) though still they have a yellow and grey bill. Shags are more strictly coastal and seldom seen inland, they also have a distinctive black crest on the top of their head.

Rock pipit (Anthus petrosus)
Size: L: 15.5-17cm
Synonymous with the coast, these small, streaked brown/grey birds (with pale underside) are commonly seen flitting from rock to rock with a swift undulating flight. They have a light peeping call and can be seen perching around harbour walls -they are often quite plucky and approachable.

Useful books and equipment:
 Seabirds: The New Identification Guide
Seabirds: The New Identification Guide
Hardback | June 2021
Lavishly illustrated with 239 full-colour plates, this is the first comprehensive guide since Harrison’s 1983 opus, covering all known seabirds, beginning with seaducks and grebes and ending with cormorants and pelicans.
 Flight Identification of European Seabirds
Flight Identification of European Seabirds
Paperback | July 2007
Containing over 650 colour photographs showing every seabird species likely to be encountered in European waters, this is an essential field guide for seawatching. Key features of each species are depicted in typical field conditions, with particular attention paid to shape and flight action, as well as plumage.
 Conservation of Marine Birds
Conservation of Marine Birds
Paperback | July 2022
This is the first book to outline and synthesize the myriad of threats faced by one of the most imperilled groups of birds on earth. With more than half of all 346 seabirds worldwide experiencing population declines, this book will be an important resource for researchers and conservationists, as well as ecologists and students.
 Gulls of Europe, North Africa, and the Middle East: An Identification Guide
Gulls of Europe, North Africa, and the Middle East: An Identification Guide
Paperback | December 2021
This title offers the most up-to-date guide for gull identification in Europe and beyond. Using a direct and visual approach, this guide provides accounts of the 45 species of gulls found int he Western Palearctic, extensively represented in nearly 1,400 colour photographs.
 Guide to Summer Coastal Birds
Guide to Summer Coastal Birds
Unbound | July 2014
From cormorants to kittiwakes, and guillemots to gulls, this 8-panel laminated fold-out chart features 28 of the birds you can see around the coastline of the UK in the summer. Birds are shown in their adult summer (breeding) plumage.
This powerful pair of image stabilising binoculars are great for use on moving boats or in unfavourable windy conditions.
These easy-to-use, entry-level binoculars have a wide field of view which, combined with their image quality, makes them great for panning.
 Opticron Explorer Compact Binoculars
Opticron Explorer Compact Binoculars
These lightweight and compact binoculars are easy to transport and store, with a weatherproof design that makes them ideal for use in the field whatever the weather.
 Swarovski CL Companion Binoculars with Wild Nature Case
Swarovski CL Companion Binoculars with Wild Nature Case
These high-end binoculars offer outstanding optical performance in a compact and lightweight body. Their wide field of view is perfect for surveying large landscapes or fast-moving animals.
 OP/TECH Bino/Cam Harness (Elastic)
OP/TECH Bino/Cam Harness (Elastic)
This self-adjusting harness reduces pressure on the neck for prolonged binocular use. The unique loop attachment system enables the harness to quickly snap in place and the binoculars to slide along the strap for use.
 Kowa TSN-500 Series Compact Spotting Scope
Kowa TSN-500 Series Compact Spotting Scope
Durable, lightweight and with excellent image quality, this compact scope is ideal for beginners or experienced birders looking for a portable alternative.
This high-end spotting scope offers a luxury viewing experience, with dual focus engineering providing a smooth, easy operation. The unparalleled image quality assures that key identification features will be easy to pick out.























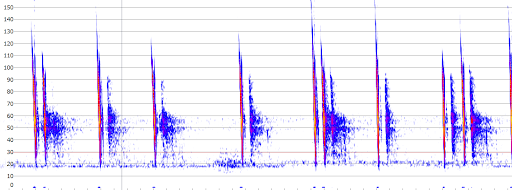
 Here we review the Anabat Scout from Titley Scientific. The Scout is an easy to use active bat detector that offers excellent quality live audio and recording, plus many handy surveying features and durability that sets it apart from other detectors. It uses heterodyne, auto-heterodyne and frequency division audio that can be recorded in either full spectrum or zero crossing files.
Here we review the Anabat Scout from Titley Scientific. The Scout is an easy to use active bat detector that offers excellent quality live audio and recording, plus many handy surveying features and durability that sets it apart from other detectors. It uses heterodyne, auto-heterodyne and frequency division audio that can be recorded in either full spectrum or zero crossing files.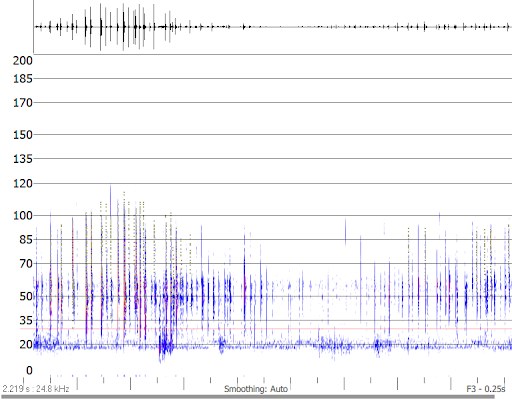



 Silvertown (plant ecologist and science writer) was the member of the New Naturalist editorial board who oversaw my book. He liked the site based approach and encouraged me to use it throughout the book (his own popular book on plant diversity ‘Demons in Eden’ had used a similar approach). In many ways the approach grew out of my tendency to ‘tell stories’ when talking about ecology to beginning undergraduates. So in part the book is my lecturing style turned to prose.
Silvertown (plant ecologist and science writer) was the member of the New Naturalist editorial board who oversaw my book. He liked the site based approach and encouraged me to use it throughout the book (his own popular book on plant diversity ‘Demons in Eden’ had used a similar approach). In many ways the approach grew out of my tendency to ‘tell stories’ when talking about ecology to beginning undergraduates. So in part the book is my lecturing style turned to prose.
 Because of the relatively early start of academic ecology in Britain the country has a number of very long running ecology field experiments. For example two I write about in the book are The Park Grass experiment at Rothamsted (started 1856) and the Godwin Plots at Wicken Fen (started 1927). Neither was started with the idea that they would run for 100 years or more – there is a large element of chance in their long-term survival. However, once an experiment has been running for a long time then people start to realise that such long runs of data are important and try and find the resources to continue them. More recent examples include (amongst many) the Buxton Climate Change Impacts Lab (which commenced in 1993 on limestone grassland in the Peak District) and grazing experiments set up in the Ainsdale Dunes system in Merseyside (started in 1974). But to be really long term requires luck and/or a succession of people determined enough to keep them going against the odds.
Because of the relatively early start of academic ecology in Britain the country has a number of very long running ecology field experiments. For example two I write about in the book are The Park Grass experiment at Rothamsted (started 1856) and the Godwin Plots at Wicken Fen (started 1927). Neither was started with the idea that they would run for 100 years or more – there is a large element of chance in their long-term survival. However, once an experiment has been running for a long time then people start to realise that such long runs of data are important and try and find the resources to continue them. More recent examples include (amongst many) the Buxton Climate Change Impacts Lab (which commenced in 1993 on limestone grassland in the Peak District) and grazing experiments set up in the Ainsdale Dunes system in Merseyside (started in 1974). But to be really long term requires luck and/or a succession of people determined enough to keep them going against the odds.


 Much of the information comes from my readings or lectures. However, since I wanted to portray the ecological and economic importance of forest insects as broadly as possible, I still had to review a lot of published material. Above all, I wanted to support quantitative data with accurate citations. Owing to the Internet, such research is easier today than it was 20 years ago… Fortunately, I also had my own photographs on almost all topics.
Much of the information comes from my readings or lectures. However, since I wanted to portray the ecological and economic importance of forest insects as broadly as possible, I still had to review a lot of published material. Above all, I wanted to support quantitative data with accurate citations. Owing to the Internet, such research is easier today than it was 20 years ago… Fortunately, I also had my own photographs on almost all topics. Professionally, I am mainly concerned with wood-dwelling insects. I am especially interested in the bark beetles, and their natural enemies as well as the intensive interactions with their host trees. Bark beetles are known to be pests, but they are also pioneers in the decay of wood. I also deal with the wood-dwelling longhorn beetles and jewel beetles, which often lend themselves to photography because of their size and beauty. For decades I have dealt with the development of their biodiversity after disruptive events such as storms or fire. The social red wood ants or the galling insects also fascinate me with their ingenious way of life.
Professionally, I am mainly concerned with wood-dwelling insects. I am especially interested in the bark beetles, and their natural enemies as well as the intensive interactions with their host trees. Bark beetles are known to be pests, but they are also pioneers in the decay of wood. I also deal with the wood-dwelling longhorn beetles and jewel beetles, which often lend themselves to photography because of their size and beauty. For decades I have dealt with the development of their biodiversity after disruptive events such as storms or fire. The social red wood ants or the galling insects also fascinate me with their ingenious way of life. There are two main causes for the decline in much of the forest insect fauna. The intensive use of wood in the past centuries has led to the fact that the forest area in Europe has decreased significantly over a long period of time, the trees no longer reach their natural age phase, and there were almost no dead trees that could slowly rot. In the case of many wood-dwelling insects that are dependent on so-called habitat trees or develop in decayed, thick tree trunks, this has led to a severe threat to their biodiversity. In recent decades, the forest area has increased again and in many countries the preservation of old trees and dead wood is being promoted. However, the impact is still modest.
There are two main causes for the decline in much of the forest insect fauna. The intensive use of wood in the past centuries has led to the fact that the forest area in Europe has decreased significantly over a long period of time, the trees no longer reach their natural age phase, and there were almost no dead trees that could slowly rot. In the case of many wood-dwelling insects that are dependent on so-called habitat trees or develop in decayed, thick tree trunks, this has led to a severe threat to their biodiversity. In recent decades, the forest area has increased again and in many countries the preservation of old trees and dead wood is being promoted. However, the impact is still modest. The main problem when photographing small objects is always to be able to focus as much as possible on them. This requires a small aperture and therefore a lot of light. I photograph everything “hand-held” and therefore the shutter speed should be short. For these reasons, I almost always use a ring flash with separately controllable halves and 100 mm macro lens with my SLR camera. Nonetheless, even cameras with a small sensor (even mobile phones!) can nowadays produce surprisingly good images of larger, less volatile insects.
The main problem when photographing small objects is always to be able to focus as much as possible on them. This requires a small aperture and therefore a lot of light. I photograph everything “hand-held” and therefore the shutter speed should be short. For these reasons, I almost always use a ring flash with separately controllable halves and 100 mm macro lens with my SLR camera. Nonetheless, even cameras with a small sensor (even mobile phones!) can nowadays produce surprisingly good images of larger, less volatile insects.









 Ecology and Natural history
Ecology and Natural history 







 Great british marine animals
Great british marine animals 










 No! I wasn’t even thinking about publishing – the journal was a personal project. I realised very early on that when I draw and document things, I remember them. I remember things like Rumex Obtusifolius (the Latin name for Doc Leaf) – it’s been a wonderful learning tool and almost everything I’ve learned about nature, I’ve learned entirely on my own through observation, supported by research.
No! I wasn’t even thinking about publishing – the journal was a personal project. I realised very early on that when I draw and document things, I remember them. I remember things like Rumex Obtusifolius (the Latin name for Doc Leaf) – it’s been a wonderful learning tool and almost everything I’ve learned about nature, I’ve learned entirely on my own through observation, supported by research.


 “In Woodland Flowers Keith Kirby invites us to look at the ‘wood beneath the trees’ and to consider what its flora can tell us. The focus of this, the eighth volume of Bloomsbury’s British Wildlife Collection (which I have contributed to myself), is on the vascular plants of the woodland floor; to this end Kirby embraces ferns as honorary flowers, but for the most part he steps aside from considering other elements of woodland ecosystems (including the ‘lower’ plants, fungi and fauna).”
“In Woodland Flowers Keith Kirby invites us to look at the ‘wood beneath the trees’ and to consider what its flora can tell us. The focus of this, the eighth volume of Bloomsbury’s British Wildlife Collection (which I have contributed to myself), is on the vascular plants of the woodland floor; to this end Kirby embraces ferns as honorary flowers, but for the most part he steps aside from considering other elements of woodland ecosystems (including the ‘lower’ plants, fungi and fauna).” “This is Sheldrake’s first book, and, while his expertise means that the readers should feel that they are in safe hands from the off, in truth the experience is more like being whisked down a burrow by a white rabbit, or on a tour of Willy Wonka’s research facility: a trippy, astonishing, and completely exhilarating ride.”
“This is Sheldrake’s first book, and, while his expertise means that the readers should feel that they are in safe hands from the off, in truth the experience is more like being whisked down a burrow by a white rabbit, or on a tour of Willy Wonka’s research facility: a trippy, astonishing, and completely exhilarating ride.” “Part autecology, part monograph and part impassioned love poem to a species that has captured the author’s heart, the pages offer an enjoyable blend of the Purple Emperor’s recorded history, biology, ecology and conservation.”
“Part autecology, part monograph and part impassioned love poem to a species that has captured the author’s heart, the pages offer an enjoyable blend of the Purple Emperor’s recorded history, biology, ecology and conservation.” “But do we really need a field guide to habitats? Possibly not. I certainly will not be taking my copy into the field. Yet this perhaps misses the point. What this book does is remind the users of other field guides that their organisms of interest do not live in isolation – they are nothing without their habitats. So, make this book an essential companion to your species guides.”
“But do we really need a field guide to habitats? Possibly not. I certainly will not be taking my copy into the field. Yet this perhaps misses the point. What this book does is remind the users of other field guides that their organisms of interest do not live in isolation – they are nothing without their habitats. So, make this book an essential companion to your species guides.” “Anyone interested in identifying and studying beetles simply cannot afford to be without [these books] and any quibbles can only be minor. Andrew cannot be too highly commended for his diligence and hard work to make so much information available to all.”
“Anyone interested in identifying and studying beetles simply cannot afford to be without [these books] and any quibbles can only be minor. Andrew cannot be too highly commended for his diligence and hard work to make so much information available to all.” “This is the latest book to enter the now relatively crowded marketplace of bumblebee guides, which may leave one wondering what it can offer to the more seasoned hymenopterist – read on! The author’s intention is to provide a book at the ‘entry level’ of bee study, Owens stating from the outset that he ‘aims to provide an easily accessible introduction for those with little or no previous knowledge of bumblebees’.”
“This is the latest book to enter the now relatively crowded marketplace of bumblebee guides, which may leave one wondering what it can offer to the more seasoned hymenopterist – read on! The author’s intention is to provide a book at the ‘entry level’ of bee study, Owens stating from the outset that he ‘aims to provide an easily accessible introduction for those with little or no previous knowledge of bumblebees’.” “There is no better place from which to view the tragi-comic events which unfold, and no better person to describe it than Derek Gow, a man of action as well as a powerful Beaver advocate. This account is unexpected, oddball, and, despite its serious side, enormously entertaining.”
“There is no better place from which to view the tragi-comic events which unfold, and no better person to describe it than Derek Gow, a man of action as well as a powerful Beaver advocate. This account is unexpected, oddball, and, despite its serious side, enormously entertaining.” “He has written an ecological masterpiece, generous in its sympathies, awe-inspiring in its breadth of knowledge, and genuinely enticing in its journey around heathland Britain. This is a book that ought to influence policy.”
“He has written an ecological masterpiece, generous in its sympathies, awe-inspiring in its breadth of knowledge, and genuinely enticing in its journey around heathland Britain. This is a book that ought to influence policy.”