The third edition of the Hazel Dormouse Conservation Handbook is based on the most recent research and practical experience available on the ecology of Hazel Dormice. It provides information on best practices for conservation, whilst a separate publication, the Hazel Dormouse Mitigation Handbook, offers further information for those involved in development projects across the UK that may affect Hazel Dormouse populations.
The third edition of the Hazel Dormouse Conservation Handbook is based on the most recent research and practical experience available on the ecology of Hazel Dormice. It provides information on best practices for conservation, whilst a separate publication, the Hazel Dormouse Mitigation Handbook, offers further information for those involved in development projects across the UK that may affect Hazel Dormouse populations.
We recently had the opportunity to speak to authors Simone Bullion, Rob Wolton and Ian White about the most recent volume, including the latest updates, how they became interested in Dormouse conservation and more.

Firstly, can you tell us a bit about yourself and how you became interested in Dormouse conservation?
Simone Bullion:
It was 25 years ago, when I had newly started a job with the Suffolk Wildlife Trust, that two events acted as a catalyst for my great interest in dormice. Firstly, I was approached by my friend, Pat Morris, to support the National Dormouse Monitoring Programme by helping to increase the number of people with dormouse licences. At the same time, there was a release of captive-bred dormice into a Suffolk woodland close to where I was living, giving me the opportunity to study them first hand. A series of funded projects followed, which helped increase our knowledge of their distribution across the county – this then initiated conservation action to restore vital linkages by planting kilometres of hedgerows. However, through time, the more I found out about dormice, the more it seemed they hadn’t read the book. Habitats that I felt quite certain would support dormice often resulted in several attempts to confirm their presence. Clearly, there were better places to nest than in the boxes and tubes I was offering them, so that led to my interest in footprint tunnels as an alternative detection method. There is still much to learn about dormice, but working with such an interesting and charismatic species has been a privilege.
Rob Wolton:
My fascination with dormice, and indeed with hedges themselves, really started when I noticed strange round nests in the hedges on our farm in Devon. As a keen birdwatcher, I could not place them. Until, one day about 30 years ago, a dormouse popped out! My first. That was the prompt that made me start to look seriously at hedges and at their wildlife, a passion that remains with me to this day. Each autumn I must admit to becoming more than a little fixated with trying to spot dormouse nests in our hedges as I walk around the farm, checking the stock or walking the dog. For several years my wife Paula took care of one, a three-legged female, no longer fit for captive breeding at Paignton Zoo. Dora, as we named her, was a huge draw, a natural lead in to talk about the farm’s wildlife and how we were encouraging it. There are few such endearing and engaging mammals.
Ian White:
I had always had an interest in conservation, but after university I worked in the retail sector. I then embarked on a new career path in my 40’s, thinking that I would become a wildlife ranger. During a training course I was asked to put together a presentation on a British mammal – I chose shrews, and so started an interest in small mammals. I was fascinated by the fact that many were considered common, but based on what? It appeared that very little was known about this group compared with larger mammals. My initial interest in dormice was somewhat biased in that, due to their conservation status, they were the only small mammal that anybody was likely to pay me for. Now after over twenty years working with dormice, I think that they are a fabulous ambassador for many other species, and they still fascinate me now as much as they did when I first saw one.

The Hazel Dormouse Conservation Handbook marks the third edition of this vital text; what can we expect from this updated volume?
Much research has been undertaken on various aspects of dormouse ecology since the release of the second Dormouse Conservation Handbook in 2006. As a result of several PhD theses and other studies, much more is known about their habitat requirements, hibernation ecology, population biology and genetics. New survey techniques are also included to aid with detection of this sometimes very elusive species.
Unlike previous editions which integrated conservation, mitigation and the effects of development within one title, the third edition presents this information into two distinct handbooks. Why was it necessary to separate this information into two guides?
Landowners, land managers and the voluntary sector continue to have a significant interest in dormouse ecology and habitat management. Separately, there is a professional interest in this protected species in the context of land use planning. Specialist knowledge has also increased, in terms of understanding best practice approaches to minimise the impacts on dormice from development. Consequently, it was decided to separate these areas of interest into two books. It is envisaged that the Hazel Dormouse Conservation Handbook will be useful to anyone with an interest in dormice, as it covers their ecology, survey techniques and habitat management. The Hazel Dormouse Mitigation Handbook will be essential as an additional and complementary reference to support the work of professional ecologists and others involved in planning.

Since the 1996 publication of the first Dormouse Conservation Handbook, how have Dormouse populations and their conservation status changed?
Sadly, Hazel Dormice have continued to decline. The ‘State of Britain’s Dormice 2023’ reported a 70% decline of dormice in monitored populations since 2000. If the observed annual rate of decline of 5.7% were to continue unabated, then dormouse counts would be expected to have fallen by more than 90% by 2034. It is also believed that there has been a loss of the species from 20 English counties over the past 100 years. Consequently, they are currently considered a vulnerable species and in danger of further localised extinction in Britain.
Which factors pose the greatest threats to dormice in the present day, and what conservation strategies are being employed in an attempt to mitigate their effects?
Whilst dormice are can live in a wide range of woody habitats, they thrive in the mid-stages of successional regrowth of woodland after coppicing, in networks of sensitively managed hedges and in scrub. However, woodland management has declined during the last 100 years; only half of our hedgerows are in favourable condition and scrub is much maligned. Dormice are also particularly vulnerable to habitat fragmentation. To be effective, dormouse conservation therefore requires landscape-scale thinking to deliver the necessary increases and improvements in their habitat, and to restore connectivity. This will also help remaining dormouse populations become more resilient to localised changes, as well as the negative impacts of unfavourable weather and climate change.
However, there are parts of their former range where natural recolonisation of dormice is extremely unlikely. The dormouse reintroduction programme, administered by the People’s Trust for Endangered Species, focuses on consolidating the current range of dormice, working to restore dormice at landscape level to create robust metapopulations.

Dormouse research relies heavily on the work of volunteer dormouse monitors, demonstrating the essential role of volunteers in conservation. What does this volunteer role entail, how does it support conservation, and how can the public get involved?
The National Dormouse Monitoring Programme (NDMP) has been running for over 25 years and is a powerful tool in monitoring population trends. As it relies on volunteers, it also provides an opportunity for people to interact with wildlife and see a dormouse close up – something that rarely happens in the wild. As dormice are a highly protected species, checking boxes for their presence must be undertaken by a trained licence holder. This training can take several years to complete, and numbers of trainees are often limited by the sites available, so sometimes there can be a waiting list to start. However, non-licence holders can also assist with putting up boxes, helping record the data and undertaking winter repairs to the boxes when they are unoccupied.
Other ways people can also get involved is to volunteer with one of your local conservation charities to help manage habitats to benefit dormice.
What do you hope the reader can learn from the Hazel Dormouse Conservation Handbook?
Dormice act as umbrella species for our native wildlife. Their habitats are home to a broad range of other species and retention of their populations is a strong indicator of habitat integrity at a landscape scale. Put simply, if we get it right for dormice, we get it right for many other species as well. We therefore hope that this handbook will help to aid the recovery of this important species.
Hazel Dormouse Conservation Handbook is available from our bookstore here.





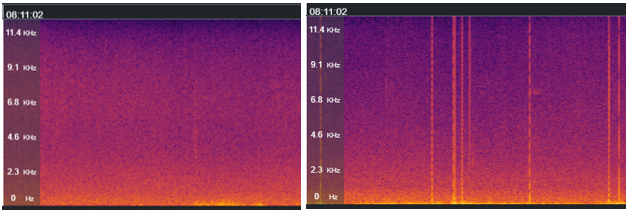
 Jan Collins has over 26 years of experience working and volunteering with bats. She has been the Head of Biodiversity at the Bat Conservation Trust for eleven years, following eleven years in ecological consultancy as a bat specialist. We recently had the opportunity to speak with Jan about the origins,
Jan Collins has over 26 years of experience working and volunteering with bats. She has been the Head of Biodiversity at the Bat Conservation Trust for eleven years, following eleven years in ecological consultancy as a bat specialist. We recently had the opportunity to speak with Jan about the origins, 


















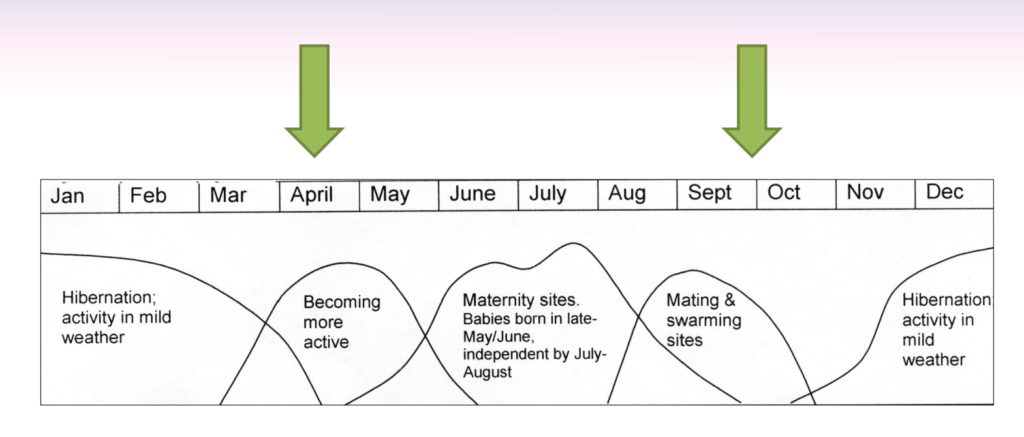














 Susan Young is a photographer and writer based in South Devon, who has a wealth of experience in wildlife photography. She has authored several books, including
Susan Young is a photographer and writer based in South Devon, who has a wealth of experience in wildlife photography. She has authored several books, including