The Mammal Society is a charity dedicated to the research and conservation of Britain’s mammals. By surveying, monitoring, researching, and sharing information about the state of mammals, it contributes to conservation efforts to help maintain these species.
The Mammal Society is a charity dedicated to the research and conservation of Britain’s mammals. By surveying, monitoring, researching, and sharing information about the state of mammals, it contributes to conservation efforts to help maintain these species.

We recently spoke to Stephanie Wray, the new Chair of Mammal Society, who kindly took the time to answer some questions about her background, her ambitions for the charity, and some ways in which you can get involved and support mammal conservation.
1. First, could you tell us a little bit about the Mammal Society and the important work that it does?
The Mammal Society is a charity which works towards the conservation of British mammals based on sound science. We were started in the 1950s and have always had both a strong academic member base of the ecologists and natural scientists who study our wild mammals, but also a fantastic body of amateur naturalists who are fascinated by mammals and willing to give up their free time to learn more about them and help their conservation in practical ways. Over time our membership has grown to include, for example, ecological consultants who work with protected species, protecting them from development, and many others who just love mammals. Increasingly we are benefiting from support from members of the public who, while they may not be able to devote time to practical projects themselves, care deeply about the British countryside and our iconic mammal species and want to help us to help them. At the moment we are developing exciting projects looking at the conservation of mountain hares, our amazing native hare which turns white in the winter and which may be threatened by climate change, and the harvest mouse, a species of traditional farmland as small as a two-pence piece and increasingly threatened by the way we manage our countryside.

2. From your PhD on brown hares in the 1990’s and a post-doc on Livingstone’s bat on the Comores, to your role as past president of the CIEEM and, most recently, your position as director of Biocensus and founder of specialist consultancy Nature Positive, you’ve had an incredibly fascinating and influential career so far. What attracted you to the position of Chair of the Mammal Society?
I’ve always had a soft spot for the Mammal Society, since my first Mammal Society conference in 1989. It’s where, a couple of years later, I presented my first scientific talk on the results of my PhD research and where I met many other mammal enthusiasts who have remained lifelong friends. Many of the Society’s members, professional and volunteer, have helped me with my research over the years, turning up in fair weather or foul to help me catch and radio-collar mammals to learn more about their habits, collect samples of dropping and other ‘glamorous’ tasks. So I want to be able to give something back, to make sure that the Society continues to grow and acts as an effective, science-backed voice for the conservation of wildlife in the UK. Our members have a huge amount of knowledge and experience and I want to make sure that we can leverage that to have our voices heard and deliver the right outcomes for conservation.
3. Unusually for an ecologist, you also have a Master’s degree in Business Administration. Do you think that marketing, economics, and the social sciences in general have an important role to play in ecology and conservation?
Well, you have done your research – I do indeed! We hear a lot about the bad things that businesses do, but the economic reach, innovation and entrepreneurship of industry can also act as a huge power for good. Businesses are starting to realise that their entire operations depend on biodiversity and that working to protect the natural world is not just a philanthropic exercise, it’s sound business sense. Sometimes how a business affects the environment is very obvious (they may use a lot of water or harvest a wild species) but in many cases it is hidden deep in their supply chains. Let’s say that you are a manufacturer of oat milk. You will have a great narrative around the climate impacts of your product compared to dairy milk, soy and almonds – but what about the biodiversity impacts? If the oats you buy are grown at a factory scale, removing hedges and ploughing up to the field boundaries, with the addition of lots of artificial fertilisers or pesticides, then you will have undone all those climate benefits through your impacts on nature – and the decline in the harvest mouse population would be an indicator of that. Now that is not just a concern to the Mammal Society – it’s a risk to your business in terms of future costs (as the environment becomes more degraded then we lose soil fertility and pollinator species, and yields will fall) in terms of your reputation (we’re drinking oat milk in the first place because we care about the environment) and in terms of your ability to attract investment (the institutional lenders don’t want to be on the wrong side of the next ‘palm oil’ issue). Under that kind of pressure, businesses can be incredibly flexible and develop new approaches, like regenerative farming, which can represent a win-win – a premium product for them, a healthier environment for everyone.

4. Taking on the role of Chair of a charity during a global pandemic must be an exciting yet challenging prospect. What are your hopes and ambitions for the charity over the next few years?
It is certainly an exciting time to take on a new role. Particularly during our first ‘lock down’ in Britain last year I think we all really appreciated our limited time outdoors and took time to enjoy those short nature ‘snacks’. For some that meant spotting wild goats or deer in the car-free streets, for others it may have been a grey squirrel or urban fox in the garden. My hope is that as we recover from the pandemic we don’t lose sight of that link to nature, and that as society moves forward it will be with increased understanding of and respect for the way the natural environment supports and underpins everything we do. My ambition for the next phase of the Mammal Society’s life is to really raise our profile to that of a household name alongside larger charities such as RSPB and WWF. I want to make sure that we develop our communications strategy, and through our website, publications and social media engagement, reach a wider audience and raise the profile of British mammals and their conservation in line with our charitable objectives. I want us to continue delivering the highest quality of scientific research and to proactively engage with government and the media on mammal conservation and management issues to contribute to the delivery of evidence-based policy.

5. As stated on your website: Britain is now recognised as one of the most nature-deprived countries in the world. Are you broadly optimistic about the future of mammals in the UK?
I think we have to be; the only viable option for human society is to live in harmony with nature. This year is a hugely important one for nature with the COP15 meeting in China in October on the post 2020 Global Biodiversity Framework, and the related COP26 meeting on climate change in Glasgow in November. These will be decisive in setting out international approaches to protected areas and sustainable use of the commodities we harvest from nature. Here in the UK, we have clear commitments arising from our exit from the EU, in the government’s 25 year environment plan, and through measures such as mandatory Biodiversity Net Gain in the forthcoming Environment Bill. All the pieces are there, we just need to commit to putting them together into a coherent protection framework. One quarter of British mammals are currently at risk, but it isn’t too late to bend the curve on extinctions and watch our biodiversity flourish again.
6. Finally, for any of our readers who are wanting to get involved with mammal conservation in the UK, what are the most important things that they can be doing right now?
Firstly (obviously!) have a look at the Mammal Society’s website (https://www.mammal.org.uk/support-us/) and you will find all sorts of things you can do to help from sending us records of mammals you have seen to organising a bake sale (hedgehog cupcakes, anyone?). In your day to day life, here are a few things you might try to help mammals.
- Garden with nature in mind. If you have a garden, try to leave a wild corner with food for wildlife particularly in the autumn and winter. An open compost heap if you have space is helpful to invertebrates, reptiles and small mammals (but don’t add any cooked food!) If you have a pond, make sure the sides are not too steep or add a ramp to make sure thirsty creatures don’t fall in and drown. Consider leaving food out for hedgehogs (cat food, never milk) or badgers (they aren’t too fussy!) if you are lucky enough to have them. Leave small gaps under fences to make sure hedgehogs and other small mammals can move around. If you don’t have a garden, a window-box or a pot of herbs on the doorstep can provide a source of food for pollinators and contribute to biodiversity.
- One of the biggest threats to nature is how we manage the countryside and as consumers we can all send a message about what we want agri-business to do. Choose products wisely – is there embedded destruction of the countryside in that breakfast cereal? Write to the supermarket or the manufacturer and ask them how they manage their impacts on biodiversity – both directly and through the ingredients they buy in.
- Write to your local MP and ask them what they will be doing to make sure that even removed from the EU’s strong environmental legislation, Britain will be a leader in environmental protection. The Wildlife and Countryside Act, which is the key piece of legislation for protecting mammal species such as bats, otters and dormice, is under review this year. Ask your MP to vote to retain and add to the strict protection we have for some mammal species and to prevent it being watered down.
 And most importantly – just go out there and watch mammals. I may be biased, but for me there is nothing better than being out in the countryside early on a spring morning watching the hares chasing around and knocking seven bells out of each other. You might like to stay up late watching badgers or bats, or enjoy the crazy antics of a squirrel on a bird feeder. It’s important that we engage with nature and encourage our children to do the same. If we don’t see and understand wildlife, we won’t fight for it. And, trust me, we need to fight for it.
And most importantly – just go out there and watch mammals. I may be biased, but for me there is nothing better than being out in the countryside early on a spring morning watching the hares chasing around and knocking seven bells out of each other. You might like to stay up late watching badgers or bats, or enjoy the crazy antics of a squirrel on a bird feeder. It’s important that we engage with nature and encourage our children to do the same. If we don’t see and understand wildlife, we won’t fight for it. And, trust me, we need to fight for it.
You can find out more about Mammal Society from their website and by following them on Facebook and Twitter.



 Formed in 2010, the core aim of the
Formed in 2010, the core aim of the  Sadly there are many threats to badgers and, despite gaining legal protection in 1992, badger persecution is on the increase. In England, one of the biggest threats badgers face is the government licenced badger cull. Since the cull began in England in 2013, a total of 140,991 badgers have been killed, 30,345 of which have been in Devon.
Sadly there are many threats to badgers and, despite gaining legal protection in 1992, badger persecution is on the increase. In England, one of the biggest threats badgers face is the government licenced badger cull. Since the cull began in England in 2013, a total of 140,991 badgers have been killed, 30,345 of which have been in Devon. In 2019, through some very generous grants and donations, the DBG was able to fund two members to train to become licenced lay vaccinators. I was lucky enough to be one of them. Since then, we have worked in collaboration with the Somerset Badger Group to vaccinate badgers in Devon and Somerset, which has allowed us to gain valuable experience. We hope this will continue and expand in the future.
In 2019, through some very generous grants and donations, the DBG was able to fund two members to train to become licenced lay vaccinators. I was lucky enough to be one of them. Since then, we have worked in collaboration with the Somerset Badger Group to vaccinate badgers in Devon and Somerset, which has allowed us to gain valuable experience. We hope this will continue and expand in the future. 





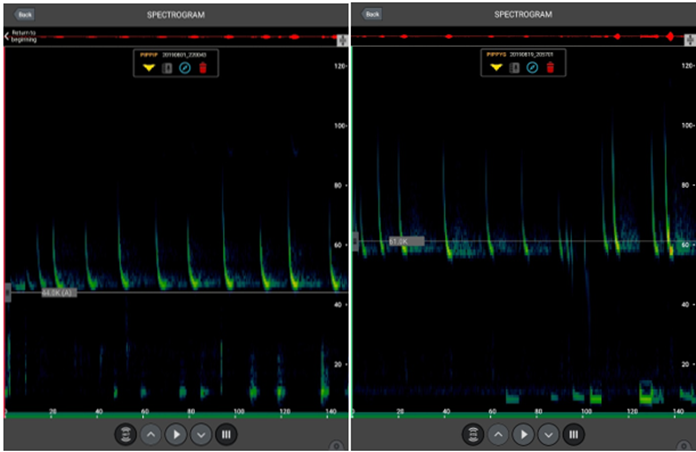
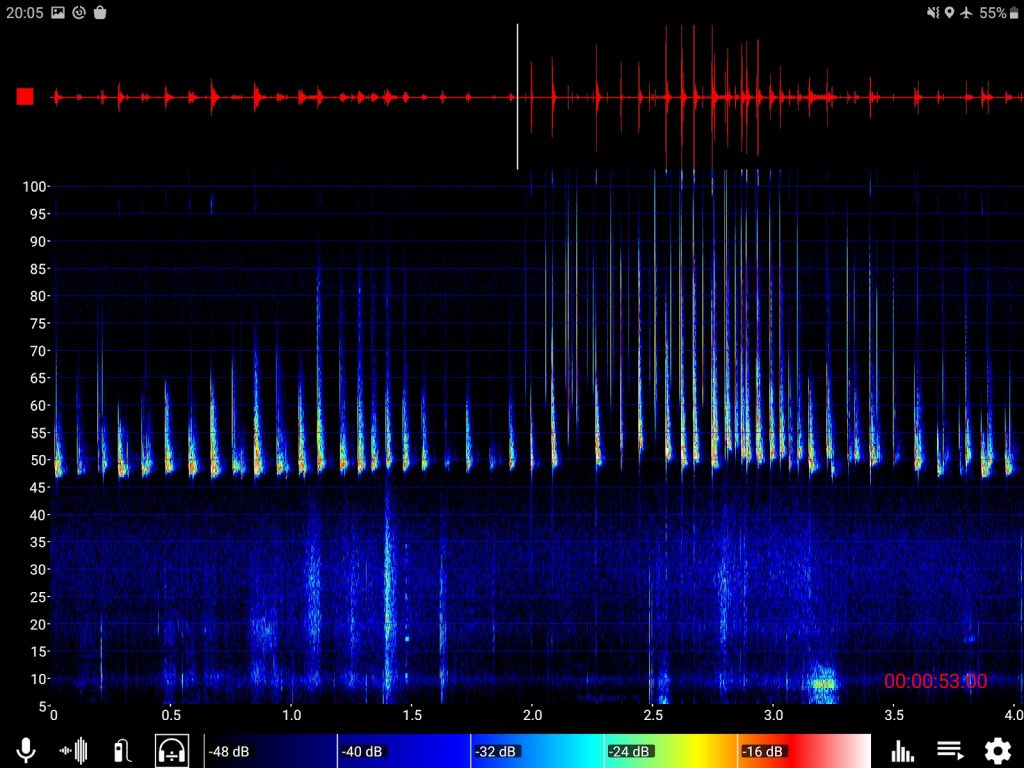
 We joined the session on thermal imaging run by Dr Kayleigh Fawcett Williams to learn more about how this equipment is being used in ecological consultancy. Most participants were new to thermal imagery, so Dr Fawcett Williams walked us through the basics. She explained that thermal imaging works by picking up thermoelectrics from the environment – infrared radiation, meaning that it is not at all invasive to the animals as the scopes emit no light (as opposed to night vision cameras that use infrared to light up the subjects).
We joined the session on thermal imaging run by Dr Kayleigh Fawcett Williams to learn more about how this equipment is being used in ecological consultancy. Most participants were new to thermal imagery, so Dr Fawcett Williams walked us through the basics. She explained that thermal imaging works by picking up thermoelectrics from the environment – infrared radiation, meaning that it is not at all invasive to the animals as the scopes emit no light (as opposed to night vision cameras that use infrared to light up the subjects).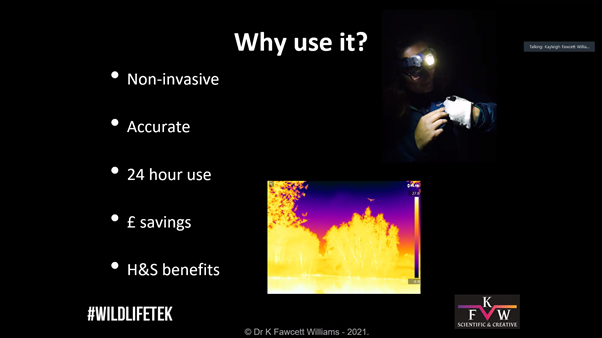 The benefits of this survey method were explained, including: ability to use at all times of day and for long durations and cost effectiveness due to lack of man power needed. Thermal imaging can reduce the risk of false positives when it comes to identification of species and also provide a wider picture of landscape or infrastructure use by illustrating patterns of activity. She also highlighted that it is good for multidisciplinary work (e.g. for firms that work with engineers) as it has a range of applications that are not just ecology based.
The benefits of this survey method were explained, including: ability to use at all times of day and for long durations and cost effectiveness due to lack of man power needed. Thermal imaging can reduce the risk of false positives when it comes to identification of species and also provide a wider picture of landscape or infrastructure use by illustrating patterns of activity. She also highlighted that it is good for multidisciplinary work (e.g. for firms that work with engineers) as it has a range of applications that are not just ecology based. She explained that bats are protected by law and that while only licensed individuals should be in contact with bats, first aid is considered emergency care and so this can be administered without a license. That being said, you will need to be able to justify any contact you have had with a bat so she strongly suggested that you keep records of the encounter.
She explained that bats are protected by law and that while only licensed individuals should be in contact with bats, first aid is considered emergency care and so this can be administered without a license. That being said, you will need to be able to justify any contact you have had with a bat so she strongly suggested that you keep records of the encounter.